A gas cylinder has the following symbol on its surface.

Which property of the gas is represented by the symbol?

Important Questions on Is Matter Around Us Pure?
Which of the following are incorrect?
i. The product formed is an element.
ii. The product cannot be classified as a compound.
iii. The product will always have a fixed composition.
iv. exhibits the properties of both A and B.
and cannot be broken down into simpler substances by simple chemical reactions. Which of the following statements concerning , and are correct?
is a compound and are electrons
and are elements have a fixed composition
Assertion: are metals because they can lose electrons to form positive ions (cations).
Reason: are non-metals because they can gain electrons to form anions.
Characteristic reactions for four different substances are given below. Arrange the tests in the order of the substances given below.
i)
ii)
iii)
iv)-
Passage through potassium dichromate turns green.
-
Dissolution in water gives a tribasic acid.
-
Non-supporter of combustion but allows active metals to continue to burn.
-
Turns alkaline pyrogallol brown.
Tiana and Prasanna were playing at home. Prasanna was playing PUBG and Tiana was doing puzzles. Mom called both of them and gave a cup of ice cream to each of them. Tiana immediately started eating ice cream, but Prasanna was playing PUBG. After some time, Tiana saw Prasanna's cup of ice cream. She observed that the ice cream started melting and also noticed that in that cup, ice cream was present in two forms, such as in liquid form and solid form. Ignoring him, she again started eating ice cream. After completing eating ice cream, she observed that all the ice cream present in Prasanna's bowl got converted into liquid. So she called Mom and told her about this thing. Then Mom took that bowl and kept it in the freezer. After some time, Mom took the ice cream bowl from the fridge and gave it to Prasanna. But the thing Tiana noticed is that liquid ice again got converted into a solid. So, she asked her Mom why ice cream changed to a liquid state when she kept it in the room, but when she kept it in the fridge again, it converted to solid. Is there any reason behind it? Then Mom said yes, substances change their state in different temperatures, so the same thing happened with the ice cream also.

Now, can you answer the below question?
When ice cream is kept in a room for some time, it changes to a liquid state. When this liquid ice cream is kept in the freezer again, it is converted into a solid. The below graph shows how the temperature of the ice cream changes with time.
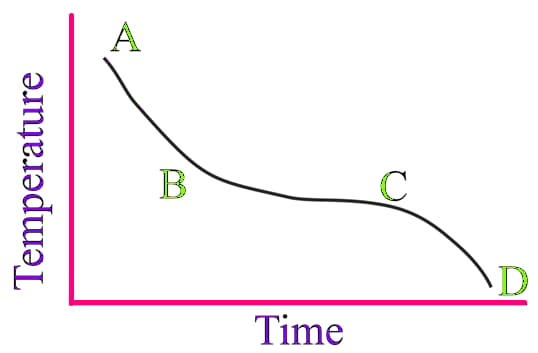
In which region of the graph both the liquid and the solid are present?
