MEDIUM
JEE Main
IMPORTANT
Earn 100
A graph showing the variation of osmotic pressure versus molar concentration of an aqueous solution at temperature is given below.
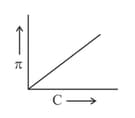
The slope of the line doesn't represent

(a)solution constant .
(b)absolute temperature .
(c)
(d)degree of ionization of solute.
50% studentsanswered this correctly

Important Questions on Solutions
MEDIUM
JEE Main
IMPORTANT
Calculate the osmotic pressure of (mass/volume anhydrous) solution at assuming ionisation in .
EASY
JEE Main
IMPORTANT
Under what conditions Van't Hoff factor is equal to unity?
EASY
JEE Main
IMPORTANT
Under what conditions van't Hoff factor is less than ?
EASY
JEE Main
IMPORTANT
Under what conditions van't Hoff factor is greater than ? Explain your answer.
MEDIUM
JEE Main
IMPORTANT
If the solution taken is too concentrated, then how will it affect the molar mass of the solute?
HARD
JEE Main
IMPORTANT
A dilute solution contains mol of solute in of a solvent with molal elevation constant the solute dimerises in solution as .
Show that the equilibrium constant for this dimer formation is , where is the elevation in boiling point for the given solution.
MEDIUM
JEE Main
IMPORTANT
The van't Hoff factor for a compound which undergoes dissociation in one solvent and association in other solvent is respectively
MEDIUM
JEE Main
IMPORTANT
The Van't Hoff's factor for a dilute aqueous solution of is
