MEDIUM
10th CBSE
IMPORTANT
Earn 100
A metal carbonate on reacting with an acid gives a gas which when passed through a solution gives the carbonate back. On the other hand, a gas that is obtained at anode during electrolysis of brine is passed on dry , it gives a compound , used for disinfecting drinking water. Identify and

Important Questions on Acids, Bases and Salts
MEDIUM
10th CBSE
IMPORTANT
A dry pellet of a common base , when kept in open absorbs moisture and turns sticky. The compound is also a by-product of chlor-alkali process. Identify , what type of reaction occurs when is treated with an acidic oxide. Write a balanced chemical equation for one such solution.
MEDIUM
10th CBSE
IMPORTANT
A sulphate salt of group 2 elements of the periodic table is a white, soft substance which can be moulded into different shapes by making its dough. When this compound is left in open for some time, it becomes a solid mass and cannot be used for moulding purpose. Identify the sulphate salt and why does it show such a behaviour? Given the reaction involved.
MEDIUM
10th CBSE
IMPORTANT
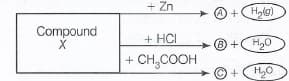
Identify the compound on the basis of the reactions given below. Also, Write the name and chemical formula of and .