A reaction follows the given concentration vs time graph. What will be the rate for this reaction at seconds?
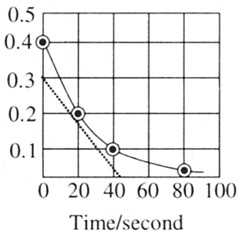


Important Questions on Chemical Kinetics
The instantaneous rate of disappearance of the ion in the following reaction is . Then, what will be the rate of appearance of ?
Reaction:
A graph of volume of hydrogen released vs time for the reaction between Zinc and dilute is given in figure. On the basis of this mark the correct statement from the following.
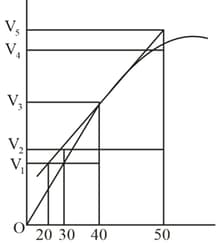
Consider the graph given in figure. Which of the following statements does not show instantaneous rate of reaction at ?

In the following reaction, which of the following are not the correct expressions regarding how the rate of appearance of the product is related to the disappearance of the reactant?
Which of the following expression(s) cannot be used to describe the instantaneous rate of the reaction ?
