A single-electron ion has a nuclear charge where is the atomic number and is electronic charge. It requires to excite the electron from the second Bohr orbit to third Bohr orbit. Find the atomic number of the element.

Important Questions on Atomic Structure
A single-electron ion has a nuclear charge where is the atomic number and is electronic charge. It requires to excite the electron from the second Bohr orbit to third Bohr orbit. Find the energy required for the transition of an electron from first to the third orbit.
A single-electron ion has a nuclear charge where is the atomic number and is electronic charge. It requires to excite the electron from the second Bohr orbit to third Bohr orbit. Find wavelength required to remove an electron from first Bohr orbit to infinity.
A single-electron ion has a nuclear charge where is the atomic number and is electronic charge. It requires to excite the electron from the second Bohr orbit to the third Bohr orbit. Find the kinetic energy of the electron in the first Bohr orbit.
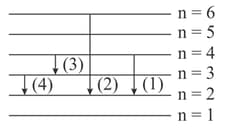
For a beam of radiation coming out from a sample of ions the following energy level diagram was observed (The beam only had photons corresponding to these transitions). If the above beam of radiations is used to excite a sample of -atoms in the ground state, then find the wavelengths of the photons subsequently emitted by the -atoms.