A thermally insulated vessel contains an ideal gas of molecular mass and ratio of specific heats . It is moving with speed and is suddenly brought to rest. Assuming no heat is lost to the surroundings, its temperature increases by :-

Important Questions on Thermodynamics
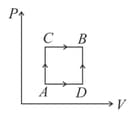
Helium gas goes through a cycle (consisting of two isochoric and isobaric lines) as shown in the figure. The efficiency of this cycle is nearly (assume the gas to be close to ideal gas)
A carnot engine, whose efficiency is , takes in heat from a source maintained at a temperature of . It is desired to have an engine of efficiency . Then, the intake temperature for the same exhaust (sink) temperature must be
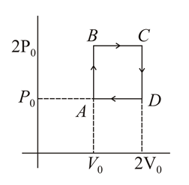
The shown diagram represents the thermodynamic cycle of an engine operating with an ideal mono atomic gas. The amount of heat extracted from the source in a single cycle is
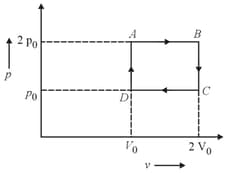
One mole of diatomic ideal gas undergoes a cyclic process as shown in the figure. The process is adiabatic. The temperature at and are , and , respectively. Choose the correct statement.
A solid body of constant heat capacity is being heated by keeping it in contact with reservoirs in two ways
(i) Sequentially keeping in contact with reservoirs such that each reservoir supplies the same amount of heat.
(ii) Sequentially keeping in contact with reservoirs such that each reservoir supplies the same amount of heat.
In both the cases, the body is brought from initial temperature to final temperature . Entropy change of the body in the two cases respectively, is
(a) the final temperature of the gas and
(b) change in its internal energy.
A gas can be taken from to via two different processes and . When path is used of heat flows into the system and of work is done by the system. If path is used work done by the system is . The heat flow into the system in path is