A vertical hollow cylinder contains an ideal gas. The gas is enclosed by a movable piston with an area of cross-section . Now, the gas is heated slowly from to and the piston rises by . The piston is now clamped at this position and the gas is cooled back to . Find the difference between the heat energy added during heating process and energy lost during the cooling process.

Important Questions on Thermodynamics
A sample of of monoatomic Helium (assumed ideal) is taken through the process and another sample of of the same gas is taken through the process as in figure. Given, molecular mass of Helium .
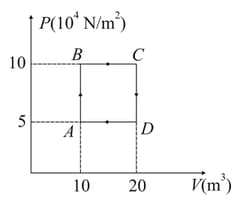
(i) What is the temperature of Helium in each of the states and ?
(ii) Is there any way of telling afterwards which sample of Helium went through the process and which went through theprocess ? Write Yes or No.
(iii) How much is the heat involved in each of the processes and .
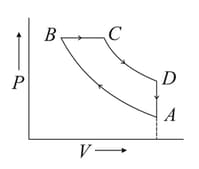
One mole of a diatomic ideal gas is taken through a cyclic process starting from point . The process is an adiabatic compression. is isobaric expansion. an adiabatic expansion and is isochoric as shown in P-V diagram. The volume ratios are and the temperature at is . Calculate the temperature of the gas at the points and and find the efficiency of the cycle.
One mole of an ideal monoatomic gas is taken around the cyclic process as shown in the figure. Calculate,
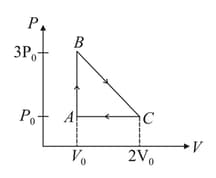
(a) the work done by the gas
(b) the heat rejected by the gas in the path and the heat absorbed by the gas in the path
(c) the net heat absorbed by the gas in the path
(d) the maximum temperature attained by the gas during the cycle.
Two moles of a monatomic gas, initially at pressure and volume , undergo an adiabatic compression until its volume becomes . Then the gas is given heat at constant volume .
(a) Sketch the complete process on a diagram.
(b) Find the total work done by the gas, the total change in its internal energy and the final temperature of the gas.
In given figure, an adiabatic cylindrical tube of volume is divided in two equal parts by a frictionless adiabatic separator. An ideal gas in left side of a tube having pressure and temperature , where as on the right side having pressure and temperature is the same for both the gases. The separator is slid slowly and is released at a position where it can stay in equilibrium. Find (a) the final volumes of the two parts, (b) the heat given to the gas in the left part and (c) the final common pressure of the gases.

