MEDIUM
NEET
IMPORTANT
Earn 100
According to Arrhenius equation, the graph of vs has slope equal to:
(a)
(b)
(c)
(d)
85.71% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
The reactant forms two products:
: Activation energy
: Activation energy
If then and will be related as:
Assume that for both the reactions, the value of Arrhenius constant is the same.
MEDIUM
NEET
IMPORTANT
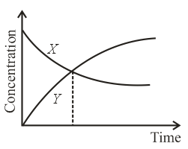
The accompanying figure depicts the change in concentration of species and for the elementary reaction as a function of time. The point of intersection of two curves represents:
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
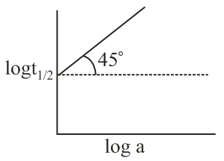
What will be the order of reaction for a chemical change having the graph between vs as shown below? where, initial concentration of reactant;
MEDIUM
NEET
IMPORTANT
