MEDIUM
JEE Main
IMPORTANT
Earn 100
According to molecular orbital theory, the species among the following that does not exist is:
(a)
(b)
(c)
(d)
75% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
JEE Main
IMPORTANT
The correct set from the following in which both pairs are in correct order of melting point is:
HARD
JEE Main
IMPORTANT
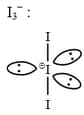
The correct shape and bond angles respectively in ion are:
MEDIUM
JEE Main
IMPORTANT
Which of the following are isostructural pairs?
A. and
B. and
C. and
D. and
MEDIUM
JEE Main
IMPORTANT
Which of the following compounds does not exhibit resonance ?
HARD
JEE Main
IMPORTANT
Match List-I with List-II
|
List - I (Elements) |
|
List - II (Properties) |
|
| (a) | |||
| (b) | |||
| (c) | |||
| (d) | |||
Choose the correct answer from the options given below:
EASY
JEE Main
IMPORTANT
The hybridisations of the atomic orbitals of nitrogen in and respectively are.
MEDIUM
JEE Main
IMPORTANT
According to the valence bond theory the hybridization of central metal atom is for which one of the following compounds?
EASY
JEE Main
IMPORTANT
The number of lone pairs of electrons on the central atom in is