An adiabatic process occurs at constant,

Important Questions on Kinetic Theory of Gases and Radiations
During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its temperature. The ratio for the gas is
If the door of a refrigerator is kept open, then which of the following is true?
If the amount of heat given to a system be and the amount of work done by the system be , then the change in the internal energy of the system is
In an adiabatic process, of work is done on the gas. The change in internal energy of the gas
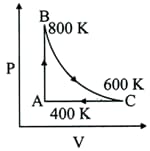
One mole of diatomic ideal gas undergoes a cyclic process as shown in the figure. The process is adiabatic. The temperatures at and are and , respectively. Choose the correct statement.
The internal energy change in a system that has absorbed of heat and done of work is similar,
If denote respectively the heat added, change in internal energy and the work done in a closed cycle process, then,
