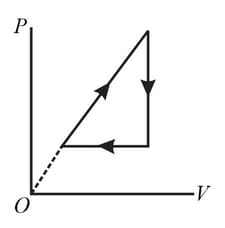
An ideal gas, whose adiabatic exponent equals , goes through a cycle consisting of two isochoric and two isobaric lines. Find the efficiency of such a cycle, if the absolute temperature of the gas rises times, both in the isochoric heating and in the isobaric expansion.

Important Questions on THERMODYNAMICS AND MOLECULAR PHYSICS
An ideal gas goes through a cycle, consisting of
(a) isochoric, adiabatic and isothermal lines,
(b) isobaric, adiabatic and isothermal lines, with the isothermal process proceeding at the minimum temperature, of the whole cycle.
Find the efficiency of each cycle, if the absolute temperature varies -folds, within the cycle.
An ideal gas goes through a cycle, consisting of
(a) isochoric, adiabatic and isothermal lines,
(b) isobaric, adiabatic and isothermal lines,
with the exception that, the isothermal process proceeds at the maximum temperature of the whole cycle.An ideal gas, with the adiabatic exponent , goes through a direct (clockwise) cycle, consisting of adiabatic, isobaric and isochoric lines. Find the efficiency of the cycle, if in the adiabatic process, the volume of the ideal gas
(a) increases -fold,
(b) decreases -fold.
Calculate the efficiency of a cycle consisting of isothermal, isobaric and isochoric lines, if in the isothermal process, the volume of the ideal gas with the adiabatic exponent ,
(a) increases -fold,
(b) decreases -fold.
An ideal gas with the adiabatic exponent , goes through a cycle within which the absolute temperature varies -fold. Find the efficiency of this cycle.