HARD
JEE Advanced
IMPORTANT
Earn 100
As shown schematically in the figure, two vessels contain water solutions (at temperature ) of potassium permanganate of different concentrations and molecules per unit volume with When they are connected by a tube of small length and cross-sectional area starts to diffuse from the left to the right vessel through the tube. Consider the collection of molecules to behave as dilute ideal gases and the difference in their partial pressure in the two vessels causing the diffusion. The speed of the molecules is limited by the viscous force on each molecule, where is a constant. Neglecting all terms of the order . Which of the following is/are correct? ( is the Boltzmann constant)
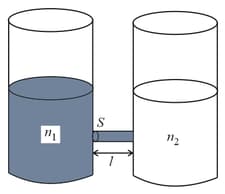

(a)the force causing the molecules to move across the tube is
(b)force balance implies
(c)total number of molecules going across the tube per sec is
(d)rate of molecules getting transferred through the tube does not change with time
15% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
MEDIUM
JEE Advanced
IMPORTANT
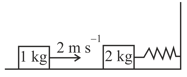
A spring - block system is resting on a frictionless floor as shown in the figure. The spring constant is and the mass of the block is . Ignore the mass of the spring. Initially the spring is in an unstretched condition. Another block of mass moving with a speed of collides elastically with the first block. The collision is such that the block does not hit the wall. The distance, in metres, between the two blocks when the spring returns to its unstretched position for the first time after the collision is _________.
HARD
JEE Advanced
IMPORTANT
A container of fixed volume has a mixture of one mole of hydrogen and one mole of helium in equilibrium at temperature . Assuming the gases are ideal, the correct statement(s) is (are)
HARD
JEE Advanced
IMPORTANT
Two non-reactive monoatomic ideal gases have their atomic masses in the ratio 2 : 3. The ratio of their partial pressures, when enclosed in a vessel kept at a constant temperature, is 4 : 3. The ratio of their densities is
