EASY
Agniveer Vayu
IMPORTANT
Earn 100
Assertion: In the process , if the volume of gas increases, then work done by gas decreases.
Reason: Work done in the above process is
(a)If both Assertion and Reason are true and the Reason is correct explanation of the Assertion.
(b)If both Assertion and Reason are true but Reason is not explanation of the Assertion.
(c)If Assertion is true but the Reason is false.
(d)If Assertion is false but Reason is true.
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
Agniveer Vayu
IMPORTANT
monoatomic gas of density and pressure is enclosed in a container. Internal energy of gas will be:
EASY
Agniveer Vayu
IMPORTANT
of a monoatomic gas is at a pressure of . The density of the gas is . What is the order of energy of the gas due to its thermal motion?
EASY
Agniveer Vayu
IMPORTANT
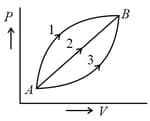
In the figure, a certain mass of gas traces three paths from state to state . If work done by the gas along three paths are respectively, then
EASY
Agniveer Vayu
IMPORTANT
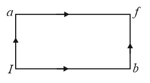
When a system is taken from state to state along the path , it is found that and . Along the path undefined . along the path is
EASY
Agniveer Vayu
IMPORTANT
An ideal gas is taken around the cycle as shown in the diagram
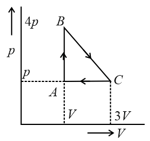
The total work done by the gas during the cycle is
EASY
Agniveer Vayu
IMPORTANT
A thermodynamical system goes from state (i) to and (ii) to . Work done in the two cases is
EASY
Agniveer Vayu
IMPORTANT
Pressure , volume and temperature of a certain material are related by where is constant. Work done by the material when temperature changes from to and pressure remains constant is
EASY
Agniveer Vayu
IMPORTANT
Assertion: The average degree of freedom per molecule for a gas is . If gas performs of work when it expands at a constant pressure then the heat absorbed by the gas is .
Reason: First law of thermodynamics is where .
