EASY
Earn 100
Assertion: Specific heat of a substance during change of state is infinite.
Reason: During change of state , specific heat does not come in.
(a)If both Assertion and Reason are true and the Reason is correct explanation of the Assertion.
(b)If both Assertion and Reason are true but Reason is not explanation of the Assertion.
(c)If Assertion is true but the Reason is false.
(d)If Assertion is false but Reason is true.
25% studentsanswered this correctly
Important Questions on Chemical Thermodynamics
EASY
EASY
MEDIUM
MEDIUM
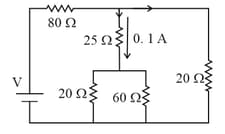
A current of flows through a resistor represented by the circuit diagram. The current in the resistor is:
EASY
EASY
EASY
HARD
A black coloured solid sphere of radius and mass is inside a cavity with a vacuum inside. The walls of the cavity are maintained at temperature . The initial temperature of the sphere is . If the specific heat of the material of the sphere varies as per unit mass with the temperature of the sphere, where is a constant, then the time taken for the sphere to cool down to temperature will be
( is Stefan Boltzmann constant)
MEDIUM
EASY
EASY
EASY
MEDIUM
[Heat of fusion of ice ; Specific heat of water ]
MEDIUM
MEDIUM
EASY
The instrument used to regulate temperature to a particular degree is called:
1. Thermostat
2. Thermometer
3. Pyrometer
4. Thermocouple
HARD
EASY
EASY
EASY
i)
ii)
iii)
iv)

