EASY
Agniveer Vayu
IMPORTANT
Earn 100
Assertion: The average degree of freedom per molecule for a gas is . If gas performs of work when it expands at a constant pressure then the heat absorbed by the gas is .
Reason: First law of thermodynamics is where .
(a)If both Assertion and Reason are true and the Reason is correct explanation of the Assertion.
(b)If both Assertion and Reason are true but Reason is not explanation of the Assertion.
(c)If Assertion is true but the Reason is false.
(d)If Assertion is false but Reason is true.
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
Agniveer Vayu
IMPORTANT
A gas can be taken from to via two different processes and .
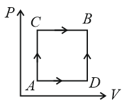
When path is used of heat flows into the system and of work is done by the system. If the path is used then work done by the system is , the heat flows into the system in the path is:
EASY
Agniveer Vayu
IMPORTANT
An ideal gas is subjected to cyclic process involving four thermodynamic states, the amounts of heat (Q) and work (W) involved in each of these states are
Q1 = 6000 J,
Q2 = -5500 J;
Q3 = -3000 J;
Q4 = 3500 J
W1 = 2500 J;
W2 = -1000 J;
W3 = -1200 J;
W4 = x J.
The ratio of the net work done by the gas to the total heat absorbed by the gas is n. The values of x and η respectively are
EASY
Agniveer Vayu
IMPORTANT
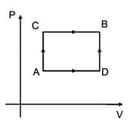
For path , Heat given to the system is and work done by the system is . For path , work done by the system is . The heat given to the system for path is
EASY
Agniveer Vayu
IMPORTANT
Five moles of an ideal monoatomic gas with an initial temperature of expand and in the process absorb of heat and does of work. The final temperature of the gas in is (ideal gas constant, ).
EASY
Agniveer Vayu
IMPORTANT
An ideal gas with adiabatic exponent undergoes a process in which work done by the gas is same as increase in internal energy of the gas. The molar heat capacity of gas for the process is
EASY
Agniveer Vayu
IMPORTANT
In the certain process, of heat are supplied to a system and at the same time of mechanical work was done on the system. The increase in its internal energy is
EASY
Agniveer Vayu
IMPORTANT
In the thermodynamical process, the pressure of a fixed mass of gas is changed in such a manner that the gas releases of heat and of work is done on the gas. If the internal energy of the gas was , then the final internal energy will be
EASY
Agniveer Vayu
IMPORTANT
The temperature of a silver bar rises by when it absorbs of energy by heat. The mass of the bar is . Determine the specific heat of silver.
