EASY
Earn 100
Assertion: Work done by a gas in reversible isothermal expansion is more than the work done by the gas in reversible adiabatic expansion if final volume is same.
Reason: Temperature remains constant in isothermal expansion, and not in adiabatic expansion.
(a)If both Assertion and Reason are true and the Reason is correct explanation of the Assertion.
(b)If both Assertion and Reason are true but Reason is not explanation of the Assertion.
(c)If Assertion is true but the Reason is false.
(d)If Assertion is false but Reason is true.
50% studentsanswered this correctly
Important Questions on Chemical Thermodynamics
EASY
Calculate the work done during compression of mol of an ideal gas from a volume of to at against a pressure of .
MEDIUM
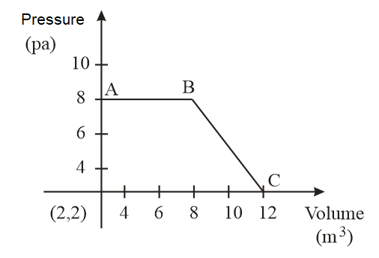
The magnitude of work done by a gas that undergoes a reversible expansion along the path shown in the figure is _________.
EASY
An ideal gas expands adiabatically against vacuum. Which of the following is correct for the given process?
MEDIUM
A thermodynamic cycle in the pressure -volume plane is given below:
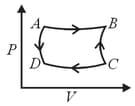
and are isothermal processes while and are adiabatic processes. The same cycle in the temperature - entropy plane is :
MEDIUM
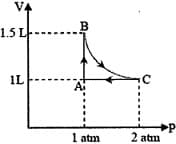
A system consisting of 1 mol of an ideal gas undergoes a reversible process, (schematically indicated in the figure below). If the temperature at the starting point A is 300 K and the work done in the process is 1 L atm, the heat exchanged in the entire process in L atm is
MEDIUM
An ideal gas initially at temperature, pressure and volume, bar and respectively is heated at constant volume until pressure is bar, it then undergoes a reversible isothermal expansion until pressure is what is the total work , during this process?
EASY
Under the isothermal condition, a gas at expands from to against a constant external pressure of bar. The work done by the gas is
(Given that bar)
MEDIUM
For water at and bar,
(Round off to the Nearest Integer)
[Use :
[Assume volume of is much smaller than volume of . Assume treated as an ideal gas]
EASY
An ideal gas undergoes isothermal expansion at constant pressure. During the process:
HARD
The volume vs. temperature graph of 1 mole of an ideal gas is given below
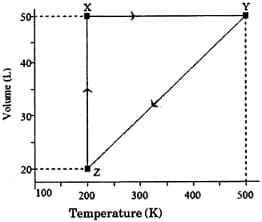
The pressure of the gas (in atm) at and respectively, are
MEDIUM
Five moles of an ideal gas at is expanded isothermally from an initial pressure of to against at constant external pressure . The heat transferred in this process is ___ . (Rounded-off to the nearest integer)
[ Use ]
HARD
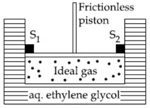
A cylinder containing an ideal gas ( of ) is in thermal equilibrium with a large volume of molal aqueous solution of ethylene glycol at its freezing point. If the stoppers and (as shown in the figure) are suddenly withdrawn, the volume of the gas in litres after equilibrium is achieved will be _____________.
(Given,
EASY
Three mole of an ideal gas expand isothermally and reversibly from to at calculate the work done?
HARD
During the free expansion of an ideal gas in an isolated chamber,
HARD
At of iron reacts with to form . The evolved hydrogen gas expands against a constant pressure of . The work done by the gas during this expansion is -_______ .
(Round off to the Nearest Integer)
[Given : . Assume, hydrogen is an ideal gas]
[Atomic mass off Fe is ]
EASY
When an ideal gas expands isothermally from to at against a constant pressure of , then the work done on the gas is
MEDIUM
The magnitude of reversible work done by an ideal gas in four different processes: isothermal expansion, adiabatic expansion, constant pressure expansion, and free expansion are , and respectively. Choose the right order of sequence for the magnitude of the work done. (Change in the volume is same for all the processes.)
EASY
If of decompose at and , the work is done () by one mole of as it expands against pressure is:
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




HARD
An ideal gas undergoes isothermal compression from to against a constant external pressure of . The heat released in this process is and is used to increase the pressure of mole of . The temperature of increases by:

