MEDIUM
Physics
IMPORTANT
Earn 100
Calculate the heat absorbed by a system in going through the cyclic process shown in the given figure.
(Take )
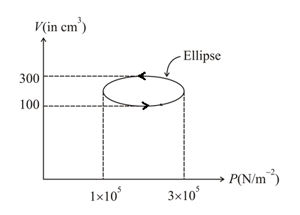

50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
Physics
IMPORTANT
gas undergoes a change of state during which of heat is supplied to it and it does of work. The system is brought back to its original state through a process during which of heat is rejected by the gas. Find the work done by the gas in the second process in joules.
EASY
Physics
IMPORTANT
Temperature of mole of an ideal gas is increased from to under isochoric process. Heat supplied to the gas in this process is . What amount of work (in joules) has to be done by the gas if temperature of the gas decreases from to adiabatically?
(Given; Gas constant )
HARD
Physics
IMPORTANT
A sample of an ideal gas is taken through a process . It absorbs of energy during the process which is an isometric process, no heat during and it rejects of heat during isobaric process . It is also given that of work is done on the gas during the process . Internal energy of gas in state is . Find the internal energy of gas in state in joules.
HARD
Physics
IMPORTANT
A gas consisting of monatomic molecules (degrees of freedom ) was expanded in a polytropic process so that the rate of collisions of the molecules against the vessel's wall did not change. Find the molar heat capacity of the gas in the process, in . (Given: Gas constant )
HARD
Physics
IMPORTANT
A gas consisting of rigid diatomic molecules was expanded polytropic process so that the rate of collisions of the molecules against the vessel's wall did not change. Find the molar heat capacity of the gas in this process in . (Given: Gas constant )
MEDIUM
Physics
IMPORTANT
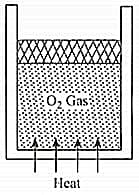
A vertically oriented cylindrical vessel containing oxygen gas and closed by a piston of mass . Piston can slide smoothly in the cylinder. Its cross-sectional area is and atmospheric pressure is . Some heat is supplied to the cylinder, so that the piston is slowly displaced up by . Find the amount of heat supplied to the gas (in Joules).
EASY
Physics
IMPORTANT
Two moles of helium gas are taken over the cycle , as shown in below in diagram.
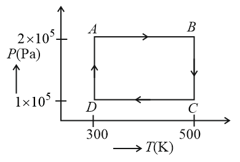
Assuming the gas to be ideal, the work done on the gas in taking it from to is
MEDIUM
Physics
IMPORTANT
Two moles of helium gas is taken over the cycle , as shown in the diagram.
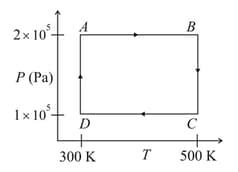
The work done on the gas in taking it from to is
