Calculate the heat of formation of acetone from the following data:
Bond energies :

Important Questions on Chemical Thermodynamics
Calculate the heat of formation of methyl alcohol (liquid) from the following data:
Heat of atomisation of
Heat of atomisation of
Heat of atomisation of
Bond energies:
Heat of liquefaction of mole of .
Calculate the heat of the following gaseous reaction:
The bond energies of and bonds are and respectively.
Estimate the heat of formation of gaseous isoprene.
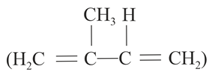
From the following data:
Bond energies:
Heat of sublimation of carbon(s) per mole.
Bond energies:
Calculate the heat of the following homogeneous gaseous reaction
from the following data:
Bond energies :
Resonance energy:
Calculate the resonance energy of from the following data:
of
Bond energies of and bonds are and respectively.
Calculate the heat of formation of from the reaction: .
