HARD
JEE Main
IMPORTANT
Earn 100
Calculate the minimum amount of energy, in joules, required to completely melt of silver initially at . [Given for silver, specific heat capacity , and latent heat of fusion , melting point ]

Important Questions on Temperature, Heat, and the First Law of Thermodynamics
HARD
JEE Main
IMPORTANT
A solid cylinder of radius length emissivity and temperature is suspended in an environment of temperature . What is the cylinder's net thermal radiation transfer rate If the cylinder is stretched until its radius is , its net thermal radiation transfer rate becomes . What is the ratio
MEDIUM
JEE Main
IMPORTANT
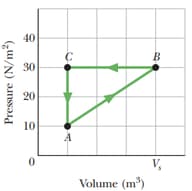
A gas within a closed chamber undergoes, the cycle shown in the diagram. The horizontal scale is set by . Calculate the net energy added to the system as a heat during one complete cycle.
HARD
JEE Main
IMPORTANT
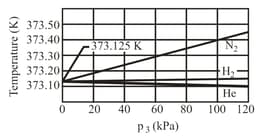
Two constant-volume gas thermometers are assembled, one with Nitrogen and the other with Hydrogen. Both contain enough gas so that .
(a) What is the difference between the pressures in the two thermometers if both the bulbs are in boiling water?
(b) Which gas is at higher pressure?
HARD
JEE Main
IMPORTANT
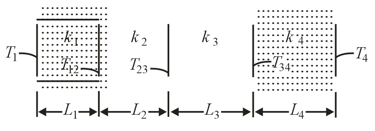
Figure shows (in cross-section) a wall consisting of four layers, with thermal conductivities and is not known). The layer thicknesses are and is not known). The known temperatures are and . Energy transfer through the wall is steady. What is interface temperature
MEDIUM
JEE Main
IMPORTANT
On a linear temperature scale, water freezes at and boils at . On a linear temperature scale, water freezes at and boils at . A temperature of corresponds to what temperature on the scale?
HARD
JEE Main
IMPORTANT
A sphere of radius temperature and emissivity is is located in an environment of temperature . At what rate does the sphere emit and absorb thermal radiation? What is the sphere's net change in energy in ?
MEDIUM
JEE Main
IMPORTANT
If you were to walk briefly in space without a spacesuit while far from the Sun (as an astronaut does in the movie A Space Odyssey), you would feel the cold of space - while you radiated energy, you would absorb almost none from your environment.
(a) At what rate would you lose energy?
(b) How much energy would you lose in ? Assume that your emissivity is and estimate other data needed in the calculations.
MEDIUM
JEE Main
IMPORTANT
Ice has formed on a shallow pond, and a steady-state has been reached, with the air above the ice at and the bottom of the pond at . If the total depth of ice and water is how thick is the ice? (Assume that the thermal conductivities of ice and water are and , respectively).
