EASY
JEE Main/Advance
IMPORTANT
Earn 100
Consider a hypothetical gas with molecules that can move along only a single axis. The following table gives four situations, the velocities in meter per second of such a gas having four molecules. The plus and minus sign refer to the direction of the velocity along the axis.

In which situation the root-mean-square speed of the molecules is greatest?

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
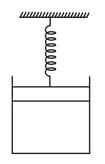
One mole of an ideal gas is kept enclosed under a light piston connected by a compressed spring (spring constant ). The volume of gas is and its temperature is . The gas is heated so that it compresses the spring further by The work done by the gas in the process is Find . (Take and suppose there is no atmosphere).
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
