MEDIUM
NEET
IMPORTANT
Earn 100
Consider the given temperature entropy diagram. Which of the following processes represent increase in volume ?
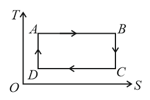

(a)DA and AB
(b)AB and BC
(c)BC and CD
(d)CD and DA
100% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
NEET
IMPORTANT
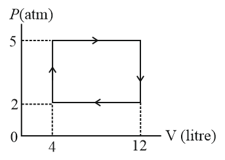
An ideal gas undergoes a cyclic process as shown in diagram. The net work done by the gas in the cycle is:-
MEDIUM
NEET
IMPORTANT
A thermodynamic system does of useful work. If its internal energy decreases by , then how much heat has been supplied to the system?
MEDIUM
NEET
IMPORTANT
In an adiabatic expansion of 0.5 mole of an ideal diatomic gas, temperature changes from 32ºC to 20ºC. The change in internal energy of the gas is
MEDIUM
NEET
IMPORTANT
A refrigerator having coefficient of performance 10 absorbs 500 J per cycle. The amount of heat rejected to the surrounding per cycle is:-
MEDIUM
NEET
IMPORTANT
The change in entropy of of nitrogen gas in an isobaric process when its temperature changes from is
EASY
NEET
IMPORTANT
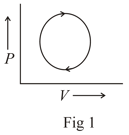
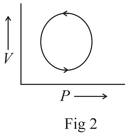
The following are the P-V diagrams for cyclic processes for a gas. In which of these processes heat is released by the gas ?
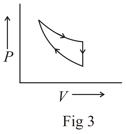
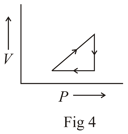
MEDIUM
NEET
IMPORTANT
A gas mixture contains and If the temperature of gas mixture is increased from to at isobaric process then find given heat of gas mixture:
MEDIUM
NEET
IMPORTANT
An ideal gas expands isothermally form a volume to and then compressed to original volume adiabatically. The initial pressure is, and the final pressure is, . The total work done is, . Then
