MEDIUM
10th CBSE
IMPORTANT
Earn 100
Copper is used in electrical appliances. Give two reasons based on its physical properties.

Important Questions on Metals and Nonmetals
MEDIUM
10th CBSE
IMPORTANT
A solution of copper sulphate cannot be stored in a vessel made of iron. Give a reason and justify it by a balanced ionic equation.
HARD
10th CBSE
IMPORTANT
Sodium metal is not refined by the electrolytic reduction of aqueous solution of sodium sulphate but copper is refined by electrolytic reduction of copper sulphate. Explain these statements in the light of the activity series of metals.
EASY
10th CBSE
IMPORTANT
Write a balanced chemical equation to describe the reaction between sodium metal and cold water. What would be observed when
(i) a burning candle is brought near the reaction mixture.
(ii) four drops of phenolphthalein are added in the reaction mixture.
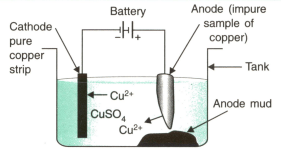
MEDIUM
10th CBSE
IMPORTANT
The given figure is the experimental setup to refine copper metal. Answer the following questions:
(i) Which material is used as anode?
(ii) In which direction do ions move in the electrolytic cell?
(iii) Name three impurities which collect as anode mud.
HARD
10th CBSE
IMPORTANT
Solutions of ferrous sulphate, zinc sulphate and silver nitrate are taken in three separate test tubes and a small strip of copper is placed in the solution of each test tube. Tell with justification in which solution or solutions will the reaction be observed?
HARD
10th CBSE
IMPORTANT
In solid state does not conduct electricity but in molten state and in aqueous solution it conducts electricity. Give separate reasons for each observation.
MEDIUM
10th CBSE
IMPORTANT
Describe the ionic bonding between potassium (atomic number) and oxygen (atomic number) in terms of electron transfer. Name the force which operates between and ions in solid .
MEDIUM
10th CBSE
IMPORTANT
An ionic compound is formed between a metal and a nonmetal. Write two important physical properties of this compound.
