Crystal systems in which no two axial lengths equal are

Important Questions on Solid State
Given is the arrangement of atoms in a crystallographic plane. Which plane correctly represent(s) the drawn structure?
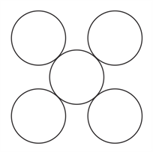
Which mineral can have the silicates skeleton as shown below?
Copper has a face-centred cubic structure with a unit-cell edge length of . What is the size of the largest atom (in pm), which could fit into the interstices of the copper lattice without distorting it?
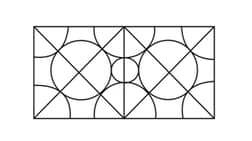
[Hint: Calculate the radius of the smallest circle in the figure.]
A metal cystallises into two cubic phases, f.c.c and b.c.c. whose unit-cell lengths are and . Calculate the ratio of densities of f.c.c. and b.c.c.
Give answer after multiplying with 100 and rounding off to the nearest integer value.
Potassium metal crystallizes in a face-centred arrangement of atoms where the edge of the unit cell is . Determine the shortest separation of any two potassium nuclei in pm is.
