Define racemic mixtures.
Important Questions on Organic Chemistry (AHL)
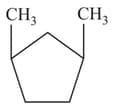
What is the total number of possible isomers of the following formula?
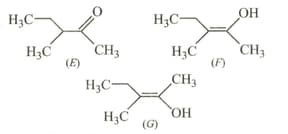
In the following structures
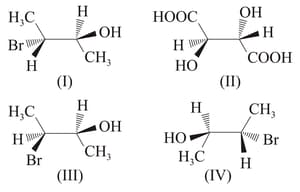
Find the correct statements.
The lowest molecular weight alkene which is optically active is/are
(a) -methyl pentene
(b) -methyl pent--ene
(c) -methyl pentene
(d) -methyl butene
(e) -methyl hex--ene
What is the maximum number of geometrical isomers possible for the following compound?
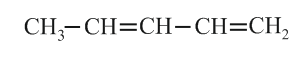
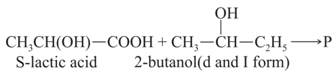
What is the solution of the product (P) formed after the reaction of with group to form ester?
How many stereoisomers of dichlorocyclohexane are optically active?
Which among the following show geometrical isomerism?
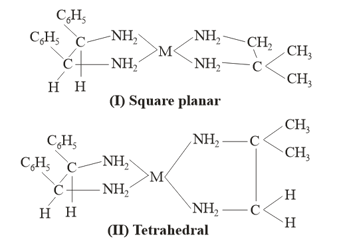
Consider the following two possible geometry of a complex around central metal and select correct statement(s) about complex.
Which of the following cycloalkanes will show cis-trans isomerism?
(a) 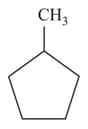
(b) 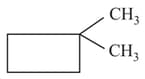
(c) 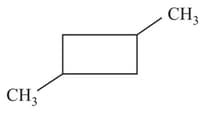
(d)