Demonstration
Electrolysis of molten zinc chloride
Safety
• This demonstration should be performed in a fume hood.
• Wear safety glasses.
• Follow correct disposal procedures.
Materials
• Large porcelain crucible
• of zinc chloride
• Tripod stand and clay-pipe triangle
• Bunsen burner
• Retort stand and clamp
• 2 carbon electrodes
• Connecting wires and crocodile clips
• 1 light bulb
• DC power supply
Method
1. Set up the apparatus as shown in the diagram.
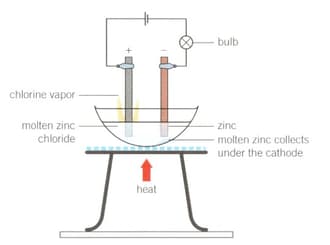
2. Light the Bunsen burner, open the gas sleeve to produce a blue roaring flame and heat continuously until a molten solution of zinc chloride is produced.
What did you observe about the light bulb as the zinc chloride solid began to melt and become molten? Support your answer with scientific reasoning.
1. Set up the apparatus as shown in the diagram.

2. Light the Bunsen burner, open the gas sleeve to produce a blue roaring flame and heat continuously until a molten solution of zinc chloride is produced.
What did you observe about the light bulb as the zinc chloride solid began to melt and become molten? Support your answer with scientific reasoning.

Important Questions on Movement
Demonstration
Electrolysis of molten zinc chloride
Safety
• This demonstration should be performed in a fume hood.
• Wear safety glasses.
• Follow correct disposal procedures.
Materials
• Large porcelain crucible
• of zinc chloride
• Tripod stand and clay-pipe triangle
• Bunsen burner
• Retort stand and clamp
• 2 carbon electrodes
• Connecting wires and crocodile clips
• 1 light bulb
• DC power supply
Method
1. Set up the apparatus as shown in the diagram.
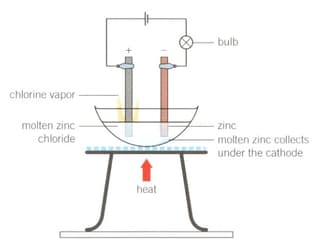
2. Light the Bunsen burner, open the gas sleeve to produce a blue roaring flame and heat continuously until a molten solution of zinc chloride is produced.
Construct balanced half-equations for the reactions occurring at the anode and the cathode, and the overall chemical equation.
Chemistry throughout the centuries
Examining the development of science and technology over the centuries gives us insight into their impact on communities, the environment, and global resources.
Joseph Wright of Derby was an influential painter of the late 18th century. This painting entitled The Alchemist: in Search of the
Philosopher’s Stone depicts a moment in time that forms a part of the history of chemistry.

This painting of Louis Pasteur in by Albert Edelfelt is in the Musée d’Orsay in Paris, France. It depicts Pasteur, a French biologist, microbiologist and chemist at work in his laboratory.

Chemistry and technology work hand in hand in the modern laboratory.

Discuss how these images portray the journey through time from alchemy to modern scientific research in the field of chemistry.
