HARD
MYP:4-5
IMPORTANT
Earn 100
Describe the physical properties of glass? Why pure glass can be considered as super viscous liquid?

Important Questions on How Do We Use Matter?
MEDIUM
MYP:4-5
IMPORTANT
If kinetic molecular theory explains the movement and arrangement of particles from solid to liquid to gas as they heat or cool. Suggest how it explains the ductility of molten glass.
MEDIUM
MYP:4-5
IMPORTANT
If kinetic molecular theory explains the movement and arrangement of particles from solid to liquid to gas as they heat or cool. How materials fuse together?
MEDIUM
MYP:4-5
IMPORTANT
If kinetic molecular theory explains the movement and arrangement of particles from solid to liquid to gas as they heat or cool. Why a slowly cooled material might be stronger than one cooled quickly.
HARD
MYP:4-5
IMPORTANT
Which of these models for solids best describes the physical properties of glass?
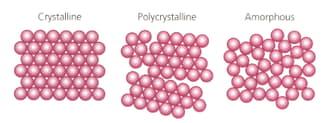
MEDIUM
MYP:4-5
IMPORTANT
MEDIUM
MYP:4-5
IMPORTANT
MEDIUM
MYP:4-5
IMPORTANT
MEDIUM
MYP:4-5
IMPORTANT
Why the oily and nonoily materials were mixed together separately before combining?
