Does the number of degrees of freedom of a gas molecule change with rise in temperature?

Important Questions on Internal Energy : First Law of Thermodynamics : Specific Heat
The figure show a graph of a gas. Calculate the work done.
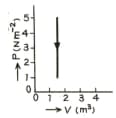
The figure show a graph of a gas. Calculate the work done.

The figure show a graph of a gas. Calculate the work done.
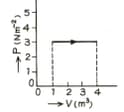
.
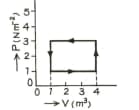
The figure show a graph of a gas. Calculate the work done.
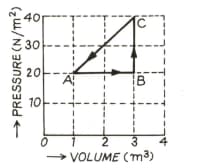
The adjoining diagram shows the pressure-volume graph of thermodynamic processes of an ideal gas. Determine the work done in processes separately and the work done in the complete cycle
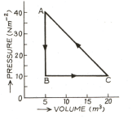
In the adjoining diagram are shown the changes taking place in a thermodynamic system in going from the initial state to the states and and finally returning to the state If joule, joule and the heat spent in the change from state to is joule, then determine work done by the system in the change from to .
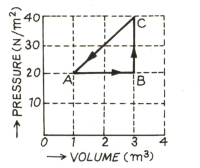
In the adjoining diagram are shown the changes taking place in a thermodynamic system in going from the initial state to the states and and finally returning to the state If joule, joule, and the heat spent in the change from state to is joule, then determine heat released from the system in the change from to .