Electroplating is an important application of electrolytic cells with commercial implications. Copper may be plated using an electrolytic cell with an aqueous acidified copper(II) sulfate electrolyte. For the copper plating of tin to make jewellery, state the half-equation at each electrode.
Assume the tin electrode is inert. Suggest two observations that you would be able to make as the electroplating progresses.

Important Questions on Redox Processes (AHL)
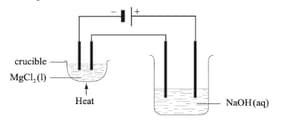
Two electrolytic cells are connected in series as shown in the diagram below. In one there is molten magnesium chloride and in the other, dilute sodium hydroxide solution. Both cells have inert electrodes. If of magnesium is produced in the first cell, deduce the identity and mass of products produced at the positive and negative electrodes in the second cell.