Experiment
What is the percentage of oxygen in atmospheric air?
Safety
• Wear gloves as steel wool can result in steel splinters entering the skin.
• Wear safety glasses.
Materials
• Steel wool
• beaker
• measuring cylinder
• Distilled water
• Retort stand and clamp
• Protective gloves
Method
1. Wedge a small, loosely packed ball of dampened steel wool into the bottom of a measuring cylinder. Ensure that it remains in place when the cylinder is inverted.
2. Record the appearance of the steel wool.
3. Place approximately of distilled water into the beaker.
4. Invert the measuring cylinder and stand it in the beaker.
5. Secure the measuring cylinder using a retort stand and clamp.
6. Record the height of the water inside the measuring cylinder using the graduations on the cylinder.
7. Leave the experiment to sit for at least one week, then measure the height of the water in the measuring cylinder again and record the appearance of the steel wool.
Describe the changes you observed in the steel wool.
1. Wedge a small, loosely packed ball of dampened steel wool into the bottom of a measuring cylinder. Ensure that it remains in place when the cylinder is inverted.

Important Questions on Movement
Experiment
What is the percentage of oxygen in atmospheric air?
Safety
• Wear gloves as steel wool can result in steel splinters entering the skin.
• Wear safety glasses.
Materials
• Steel wool
• beaker
• measuring cylinder
• Distilled water
• Retort stand and clamp
• Protective gloves
Method
1. Wedge a small, loosely packed ball of dampened steel wool into the bottom of a measuring cylinder. Ensure that it remains in place when the cylinder is inverted.
2. Record the appearance of the steel wool.
3. Place approximately of distilled water into the beaker.
4. Invert the measuring cylinder and stand it in the beaker.
5. Secure the measuring cylinder using a retort stand and clamp.
6. Record the height of the water inside the measuring cylinder using the graduations on the cylinder.
7. Leave the experiment to sit for at least one week, then measure the height of the water in the measuring cylinder again and record the appearance of the steel wool.
Calculate the percentage change in the amount of air in the measuring cylinder.
Experiment
What is the percentage of oxygen in atmospheric air?
Safety
• Wear gloves as steel wool can result in steel splinters entering the skin.
• Wear safety glasses.
Materials
• Steel wool
• beaker
• measuring cylinder
• Distilled water
• Retort stand and clamp
• Protective gloves
Method
1. Wedge a small, loosely packed ball of dampened steel wool into the bottom of a measuring cylinder. Ensure that it remains in place when the cylinder is inverted.
2. Record the appearance of the steel wool.
3. Place approximately of distilled water into the beaker.
4. Invert the measuring cylinder and stand it in the beaker.
5. Secure the measuring cylinder using a retort stand and clamp.
6. Record the height of the water inside the measuring cylinder using the graduations on the cylinder.
7. Leave the experiment to sit for at least one week, then measure the height of the water in the measuring cylinder again and record the appearance of the steel wool.
Explain how the percentage change in the level of air in the measuring cylinder compares with the percentage of oxygen in atmospheric air.
Experiment
What is the percentage of oxygen in atmospheric air?
Safety
• Wear gloves as steel wool can result in steel splinters entering the skin.
• Wear safety glasses.
Materials
• Steel wool
• beaker
• measuring cylinder
• Distilled water
• Retort stand and clamp
• Protective gloves
Method
1. Wedge a small, loosely packed ball of dampened steel wool into the bottom of a measuring cylinder. Ensure that it remains in place when the cylinder is inverted.
2. Record the appearance of the steel wool.
3. Place approximately of distilled water into the beaker.
4. Invert the measuring cylinder and stand it in the beaker.
5. Secure the measuring cylinder using a retort stand and clamp.
6. Record the height of the water inside the measuring cylinder using the graduations on the cylinder.
7. Leave the experiment to sit for at least one week, then measure the height of the water in the measuring cylinder again and record the appearance of the steel wool.
Write a balanced chemical equation to describe the reaction that has taken place between the element iron and oxygen.
Experiment
What is the percentage of oxygen in atmospheric air?
Safety
• Wear gloves as steel wool can result in steel splinters entering the skin.
• Wear safety glasses.
Materials
• Steel wool
• beaker
• measuring cylinder
• Distilled water
• Retort stand and clamp
• Protective gloves
Method
1. Wedge a small, loosely packed ball of dampened steel wool into the bottom of a measuring cylinder. Ensure that it remains in place when the cylinder is inverted.
2. Record the appearance of the steel wool.
3. Place approximately of distilled water into the beaker.
4. Invert the measuring cylinder and stand it in the beaker.
5. Secure the measuring cylinder using a retort stand and clamp.
6. Record the height of the water inside the measuring cylinder using the graduations on the cylinder.
7. Leave the experiment to sit for at least one week, then measure the height of the water in the measuring cylinder again and record the appearance of the steel wool.
Identify the type of reaction that iron has undergone.
Demonstration
Electrolysis of molten zinc chloride
Safety
• This demonstration should be performed in a fume hood.
• Wear safety glasses.
• Follow correct disposal procedures.
Materials
• Large porcelain crucible
• of zinc chloride
• Tripod stand and clay-pipe triangle
• Bunsen burner
• Retort stand and clamp
• 2 carbon electrodes
• Connecting wires and crocodile clips
• 1 light bulb
• DC power supply
Method
1. Set up the apparatus as shown in the diagram.
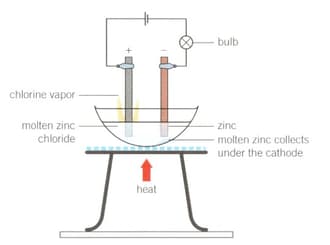
2. Light the Bunsen burner, open the gas sleeve to produce a blue roaring flame and heat continuously until a molten solution of zinc chloride is produced.
What did you observe about the light bulb as the zinc chloride solid began to melt and become molten? Support your answer with scientific reasoning.
Demonstration
Electrolysis of molten zinc chloride
Safety
• This demonstration should be performed in a fume hood.
• Wear safety glasses.
• Follow correct disposal procedures.
Materials
• Large porcelain crucible
• of zinc chloride
• Tripod stand and clay-pipe triangle
• Bunsen burner
• Retort stand and clamp
• 2 carbon electrodes
• Connecting wires and crocodile clips
• 1 light bulb
• DC power supply
Method
1. Set up the apparatus as shown in the diagram.
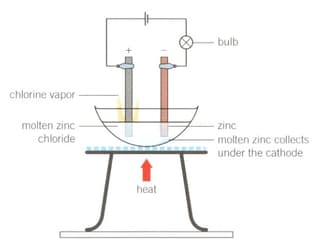
2. Light the Bunsen burner, open the gas sleeve to produce a blue roaring flame and heat continuously until a molten solution of zinc chloride is produced.
Construct balanced half-equations for the reactions occurring at the anode and the cathode, and the overall chemical equation.
