EASY
Earn 100
Explain how a polarimeter can be used to identify enantiomers?
Important Questions on Organic Chemistry (AHL)
HARD
For the given compound X, the total number of optically active stereoisomers is ____.
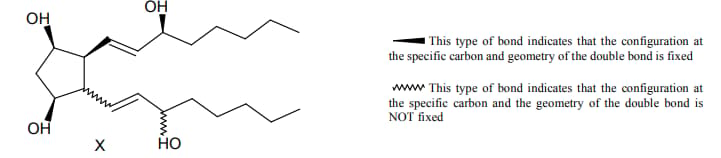
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
I. Trans-1-chloro-2-methylcyclopropane
II. Cis-1-chloro-2-methylcyclopropane
III. 1-chloro-1-methylcyclopropane
IV. Cis-1, 2-dichlorocyclopropane
HARD
EASY
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
MEDIUM
EASY
HARD
Trans-3,6-Dimethyl oct-4-ene (A) exists in two diastereomers and .
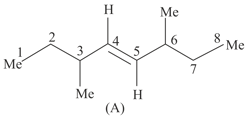
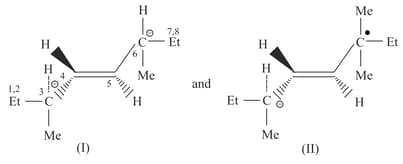
Which statements is true about and ?
MEDIUM
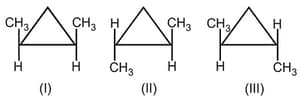
HARD
HARD
MEDIUM
(+) - mandelic acid has a specific rotation of 158o. What would be the observed specific rotation of a mixture of 25% (-) - mandelic acid and 75% (+) - mandelic acid ?
MEDIUM


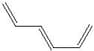 the double bonds are
the double bonds are