Extraction of highly electropositive metal is done by
electrolysis of aqueous solution of metal chloride.
electrolysis of molten metal chloride.
carbon reduction of the oxide of the metal.
strongly heating the oxide of the metal.

Important Questions on Metals And Non-metals
Some properties of substances P, Q, R and S are given in the table:
| Substance | Melting point, | Boiling point, | Electrical conductivity |
| P | Good | ||
| Q | Poor | ||
| R | Poor | ||
| S | Good |
Which of the given substances represents a gaseous non-metal at room temperature?
Match column-I with column-II and select the correct option using the given codes.
| Column I | Column II |
| P. A metal that forms two types of oxides | (i) |
| Q. A metal used in hot water apparatus | (ii) |
| R. A metal which can displace hydrogen from steam only | (iii) |
| S. A metal that does not react with air even at high temperature | (iv) |
Ms. Neelam, a class teacher took and strips respectively in four test tubes labelled as I, II, III and IV and added of freshly prepared ferrous sulphate solution to each test tube as shown in the figure:

Black residue would be obtained in test tubes
A few chemical properties of three metals X, Y and Z are summarised in the given table.
| Metal | Chemical Properties |
| X | Reacts with hot water and starts floating on the surface of water. |
| Y | Reacts with steam. |
| Z | Does not react with dilute acids as well as steam. |
The methods which can be used to extract X, Y and Z from their ores are respectively,
Mitaali, a class student wanted to study the speed of reactions of different acids with zinc metal. For this, she conducted the following experiments using zinc metal in excess.
| Experiment | Acid Used |
| I. | of . |
| II. | of . |
| III. | of . |
She measured the volume of gas produced at regular time intervals and summarised her results in a graphical form. Which of the following graphs best represents the results obtained in experiments I, II and III?
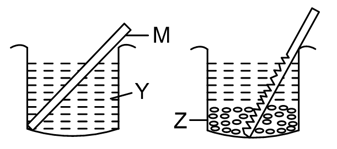
A metal rod M was dipped in a coloured solution Y. After some time it was observed that the metal rod starts dissolving in the solution and the solution starts fading in colour. However, a coloured precipitate Z was seen at the bottom of the beaker. M, Y and Z could be