Figure 28.20 shows another energy level diagram. In this case, energy is given in electronvolts (eV). The list shows the energies of some photons: . State and explain which of these photons will be absorbed by the electrons.
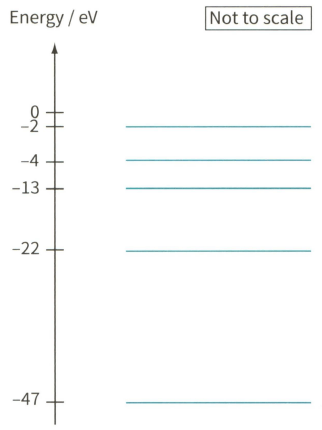
An energy level diagram.

Important Questions on Quantum Physics
The line spectrum for a particular type of atom is found to include the following wavelengths: . Calculate the corresponding photon energies in eV.
The line spectrum for a particular type of atom is found to include the following wavelengths: 83 nm 50 nm 25 nm.
(b) Sketch the energy levels that could give rise to these photons. On the diagram, indicate the corresponding electron transitions responsible for these three spectral lines.
X-rays are used to find out about the spacings of atomic planes in crystalline materials. Describe how beams of electrons could be used for the same purpose.
X-rays are used to find out about the spacings of atomic planes in crystalline materials. How might electron diffraction be used to identify a sample of a metal?
A beam of electrons is accelerated from rest through a p.d. of . What is the energy (in eV) of each electron in the beam?
A beam of electrons is accelerated from rest through a p.d. of .
Calculate the speed, and hence the momentum , of each electron.
A beam of electrons is accelerated from rest through a p.d. of .
(c) Calculate the de Broglie wavelength of each electron.
A beam of electrons is accelerated from rest through a p.d. of . Would you expect the beam to be significantly diffracted by a metal film in which the atoms are separated by a Spacing of .
