EASY
Agniveer Vayu
IMPORTANT
Earn 100
Find the percentage decrease in the volume if the pressure of an ideal gas is increased by at constant temperature.
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
Agniveer Vayu
IMPORTANT
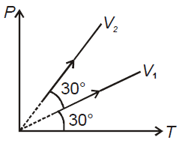
graph for same amount of an ideal gas is allowed to expand as shown in the figure below. Then which of the following is right?
EASY
Agniveer Vayu
IMPORTANT
Two gases having same pressure and volume are mixed at a temperature . If the mixture is at a temperature and occupies the same volume then pressure of the mixture would be
EASY
Agniveer Vayu
IMPORTANT
You are riding on your bicycle with inflated tyres. Your friend asks for a lift and sits on the carrier behind you
EASY
Agniveer Vayu
IMPORTANT
Two soap bubbles of radii and coalesce to constitute a bubble of radius . Then is equal to
EASY
Agniveer Vayu
IMPORTANT
Which type of molecular motion does not contribute towards internal energy:-
EASY
Agniveer Vayu
IMPORTANT
Among the following statements, the correct statement is
EASY
Agniveer Vayu
IMPORTANT
A gas for which is suddenly compressed to the th of the initial volume. Then the ratio of the final to the initial pressure is
EASY
Agniveer Vayu
IMPORTANT
For an ideal gas, the specific heat at constant pressure is greater than the specific heat at constant volume . This is because
