For a consecutive first-order reaction the values of and are and , respectively. If the reaction is carried out with pure at a concentration of , what will be the composition of the reacting system after a time interval of minutes?

Important Questions on Chemical Kinetics
The formation in water of -potassium chromo-oxalate from its -form is a reversible reaction, which is first-order in both directions, the racemate being the equilibrium product. A polarimeter experiment at showed that after of the -isomer was converted to the -form. Find the rate constant for the forward and the reverse reactions.
Given
Derive the equilibrium constant for decomposition of from the following data
| Time in minute | |||||
| Volume of given by |
The kinetics of hydrolysis of methyl acetate in excess dilute at were followed by withdrawing of the reaction mixture at intervals of , adding water, and titrating with baryta water. Determine the velocity constant of hydrolysis.
| (in a minute) | |||||
| Titre value (in ) |
In the inversion of cane sugar in the presence of an acid, the following polarimeter readings are obtained.
| Time (in minute) | |||||
| Rotation (in degree) |
Calculate the rate constant.
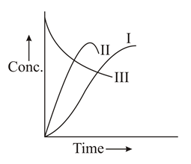
For the consecutive unimolecular-type first-order reaction the concentration of component at any time is given by
if at ,
The time at which the maximum concentration of occurs is
In a , a homogeneous reaction's first-order of the type (consecutive reaction) is carried out. Among the following, which curve shows a variation of concentration of and with time?