For a gas sample with number of molecules, the function is given by, for and for . Where is a number of molecules in speed range to . The rms speed of the molecules is

Important Questions on Kinetic Theory of Gases
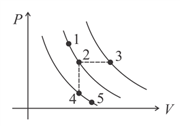
A certain gas is taken to the five states represented by dots in the graph. The plotted lines are isotherms. The order of the most probable speed of the molecules at these five states is,
One mole of an ideal gas undergoes a process in which, , where and are positive constants and is volume. The minimum pressure attainable is,
The volume thermal expansion coefficient of an ideal gas at constant pressure is
(Here absolute temperature of gas)
Consider a hypothetical gas with molecules that can move along only a single axis. The following table gives four situations, the velocities in meter per second of such a gas having four molecules. The plus and minus sign refer to the direction of the velocity along the axis.

In which situation the root-mean-square speed of the molecules is greatest?
