Four groups of students in Mrs Kiran's class were investigating how the mass of magnesium changes when it burns. Burning is a phenomenon where a substance reacts with oxygen. They noted that it burns with a white sparkling flame similar to the sparks we see when we burn crackers.
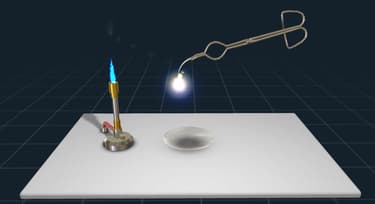
At the end of the experiment, they noted the initial mass of magnesium before burning and the mass of magnesium at the end of burning. These data are shown below in the table.
Group
Mass of magnesium in the starting
Mass of magnesium at the end
- Three groups analysed the increase in the mass of the metal at the end of the reaction. Could you state the reason for this increase? Also, mention whether the reaction is exothermic or endothermic.
- Which group has not performed the experiment correctly?
- Which law of chemical reaction can be explained with the help of this experiment? What other data is needed to prove the law correctly.



Important Questions on Chemical Reactions and Equations
Mrs Brown’s class were investigating how the mass of magnesium changes when it burns.
At the end of the experiment, they collected the results together in a table.
| Group | Mass of magnesium in the starting | Mass of magnesium at the end |
- Explain which group’s result was unexpected. Give a reason for your answer.
(a) What do you think is metal X?
(b) What could be gas Y2?
(c) What is compound XO?
(d) What is compound Y2O?
(e) Write the chemical equation of the reaction which takes place on heating XO with Y2.
(f) What type of chemical reaction is illustrated in the above equation?
Anthracite contains carbon and generally has the highest heating value of all ranks of coal. It burns to give heat and light, thus, it is used as a fuel. This heat energy is widely used in power generation and in steel industries. Coal is used for household purposes since early times. Taking the ideal condition for coal that it is pure carbon with no impurities, such a coal when burnt will liberate carbon dioxide gas along with heat energy.

- If coal is represented by a chemical symbol , write the reaction for the complete combustion of coal.
- Could you explain the type of reaction given in part one?
- Can this reaction be considered as an oxidation reaction? If yes, explain why?
