HARD
Upper Secondary-IGCSE
IMPORTANT
Earn 100
Graphite is one of the crystalline forms of carbon. Two of the distinctive properties of graphite are:
- It conducts electricity even though it is a non-metal, and
- It can act as a lubricant even though it has a giant covalent structure.
Give a brief explanation of these properties in light of the structure of graphite.
Graphite as an electrical conductor.
Give a brief explanation of these properties in light of the structure of graphite.

Important Questions on Elements and Compounds
HARD
Upper Secondary-IGCSE
IMPORTANT
Graphite is one of the crystalline forms of carbon. Two of the distinctive properties of graphite are:
- It conducts electricity even though it is a non-metal, and
- It can act as a lubricant even though it has a giant covalent structure.
Give a brief explanation of these properties in light of the structure of graphite.
Graphite are as lubricant.
HARD
Upper Secondary-IGCSE
IMPORTANT
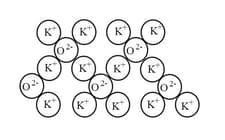
What is the ratio of K+ ions to O2-ions? _____ .
HARD
Upper Secondary-IGCSE
IMPORTANT
The diagram below shows a representation of the structure of an ionic oxide.
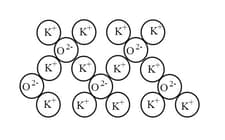
What is the formula of this compound?
HARD
Upper Secondary-IGCSE
IMPORTANT
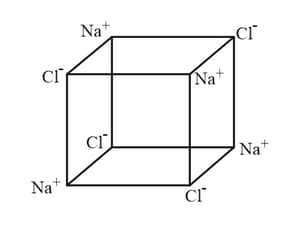
Extend the structure to the right, by adding four more ions.
HARD
Upper Secondary-IGCSE
IMPORTANT
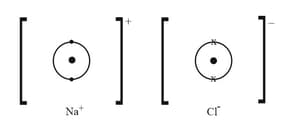
HARD
Upper Secondary-IGCSE
IMPORTANT
HARD
Upper Secondary-IGCSE
IMPORTANT
Draw dot and cross structural diagram to represent the bonding in the following simple molecular compounds. In the dot and cross diagram, show only the outer shells of the atoms involved.
| Molecule | Dot and Cross diagram | Structure |
| Ammonia | ||
| Water | ||
| Hydrogen chloride | ||
| Ethane | ||
| Ethene | ||
| Ethanol | ||
| Ethanoic acid |
MEDIUM
Upper Secondary-IGCSE
IMPORTANT
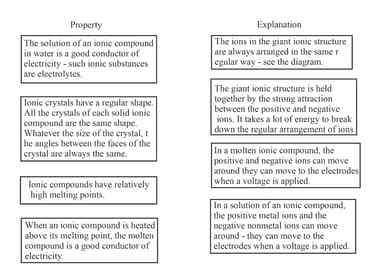
The boxes below contain properties of ionic compounds and their explanations.Draw lines to link each pair.