MEDIUM
NEET
IMPORTANT
Earn 100
is a tribasic acid and one of its salt is . What volume of solution should be added to of to convert it into ?
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Redox Reactions
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
Which are not in balanced position?
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
The equivalent weight of nitric acid in the above reaction is
MEDIUM
NEET
IMPORTANT
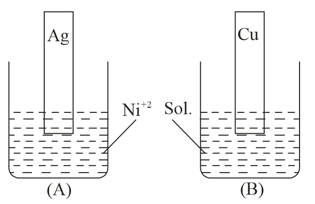
Two beakers, and each contain a nickel ion solution. A silver strip is dipped in beaker and a copper strip is dipped in beaker .
Given that,
and .
Based on the above information, which of the following is correct?
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
a)
b)
c)
Based on the data given above, what is the correct order of reducing power?