HARD
JEE Main
IMPORTANT
Earn 100
ions are in an excited state from where maximum types of photons can be emitted. If is the separation energy of from this excited state, then how many times of energy is required to ionise hydrogen atom?

Important Questions on Atomic Structure
MEDIUM
JEE Main
IMPORTANT
Separation energy of a hydrogen-like ion from its third excited state is times the separation energy of hydrogen atom from its first excited state. Find out the atomic number of hydrogen-like ion.
HARD
JEE Main
IMPORTANT
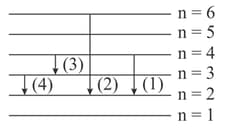
For a beam of radiation coming out from a sample of ions the following energy level diagram was observed (The beam only had photons corresponding to these transitions). If the above beam of radiations is used to excite a sample of -atoms in the ground state, then find the wavelengths of the photons subsequently emitted by the -atoms.
MEDIUM
JEE Main
IMPORTANT
What is the difference in the wavelengths of and lines of Balmer series in the spectrum of a hydrogen atom?
MEDIUM
JEE Main
IMPORTANT
Two electrons are revolving around a nucleus at distances and The ratio of their time periods is:
HARD
JEE Main
IMPORTANT
Which of the following is not correct for the velocity of electron?
HARD
JEE Main
IMPORTANT
The radii of two of the first four Bohr orbits of the hydrogen atom are in the ratio The energy difference between them may be
MEDIUM
JEE Main
IMPORTANT
Which is the correct relationship?
MEDIUM
JEE Main
IMPORTANT
In a certain electronic transition in the hydrogen atoms from an initial state to the final state , the difference in the orbital radius is times the first Bohr radius. Identify the transition.
