Heat capacity is an extensive property but specific heat is an intensive property. What will be the relation between and for mol of water?

Important Questions on Thermodynamics
The net enthalpy change of a reaction is the amount of energy required to break all the bonds in reactant molecules minus amount of energy required to form all the bonds in the product molecules. What will be the enthalpy change for the following reaction.
Given that Bond energy of and is and respectively.
The enthalpy of reaction for the reaction:
is . What will be standard enthalpy of formation of ?
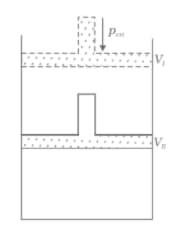
What will be the work done on an ideal gas enclosed in a cylinder, when it is compressed by a constant external pressure, in a single step as shown in Figure? Explain graphically.
Represent the potential energy/enthalpy change in the following processes graphically.
(a) Throwing a stone from the ground to roof.
(b)
In which of the processes potential energy/enthalpy change is contributing factor to the spontaneity?
