Hydrazine, , is a weak base. Write a chemical equation to show the equilibrium reaction of hydrazine with water.

Important Questions on Equilibria
The of a solution depends on the hydrogen ion (hydroxonium ion) concentration. Which concentration of ethanoic acid in Table has the highest concentration of hydrogen ions in solution?
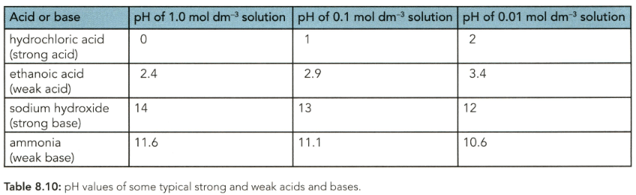
Which acid or alkali in Table has the highest concentration of hydroxide ions?

Both hydrochloric acid and ethanoic acid react with magnesium. The rate of reaction of hydrochloric acid with magnesium is much faster than the rate of reaction of ethanoic acid. Explain why.
Use Table to identify: those indicators which could be used for a strong acid-strong base titration like the one in Figure .
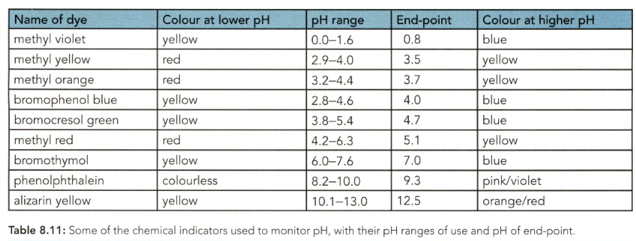
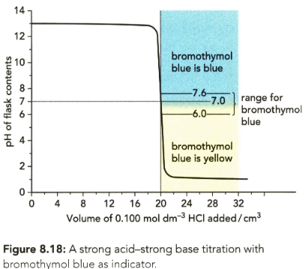
Use Table to identify: the indicators that could not be used for the strong acid-strong base titration.
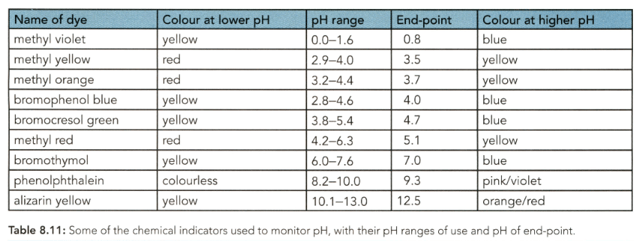
Suggest a suitable indicator to find the end-points of the reactions between: nitric acid and aqueous ammonia.
