MEDIUM
JEE Main
IMPORTANT
Earn 100
Hydrogenation of vegetable ghee at reduces the pressure of from to in Calculate the rate of reaction in terms of change in pressure per minute.

Important Questions on Chemical Kinetics
MEDIUM
JEE Main
IMPORTANT
Hydrogenation of vegetable ghee at reduces the pressure of from to in Calculate the rate of reaction in terms of change in molarity per second.
MEDIUM
JEE Main
IMPORTANT
In a reaction , the concentration of decreases from to in Calculate the average rate during this interval in .
MEDIUM
JEE Main
IMPORTANT
A drop of solution (volume ) contains of . If the rate constant of disappearance of is . How long would it take for in the drop to disappear?
MEDIUM
JEE Main
IMPORTANT
In a gaseous state reaction, . The increase in pressure from to is noticed in The rate of disappearance of in is
HARD
JEE Main
IMPORTANT
For a gas reaction at , the rate is given by . If the rate equation is expressed as ,, the rate constant is given by
(where, ideal gas law constant, )
HARD
JEE Main
IMPORTANT
In the following reaction ,
.
where '' sign indicates rate of disappearance of the reactant. Thus, is
MEDIUM
JEE Main
IMPORTANT
A reaction follows the given concentration vs time graph. What will be the rate for this reaction at seconds?
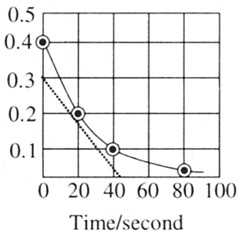
MEDIUM
JEE Main
IMPORTANT
The instantaneous rate of disappearance of the ion in the following reaction is . Then, what will be the rate of appearance of ?
Reaction:
