EASY
NEET
IMPORTANT
Earn 100
If system is in thermal equilibrium with and is separately in thermal equilibrium with then and are in thermal equilibrium. From which thermodynamics law, does this follow?
(a)Zeroth
(b)First
(c)Second
(d)Third
100% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
If ideal diatomic gas follows the process as shown in graph where is temperature in and is volume , then molar heat capacity for this process will be [in terms of gas constant ],
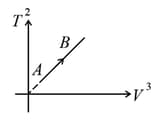
MEDIUM
NEET
IMPORTANT
A thermodynamic system is taken through the cycle as shown in the figure. Heat rejected by the gas during the cycle is:
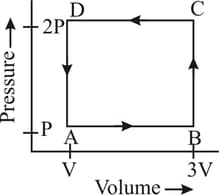
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
Helium gas goes through a cycle (consisting of two isochoric and isobaric lines) as shown in figure. Efficiency of this cycle is nearly (Assume the gas to be close to ideal gas)
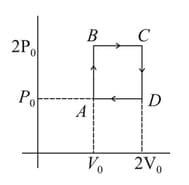
MEDIUM
NEET
IMPORTANT
A Carnot engine takes of heat from a reservoir at and gives it to a sink at . The work done by the engine is,
EASY
NEET
IMPORTANT
