MEDIUM
Chemistry
IMPORTANT
Earn 100
If the three inter-axial angles defining the unit cell are all equal in magnitude, then the crystal cannot belong to the
(a)orthorhombic system.
(b)cubic system.
(c)hexagonal system.
(d)triclinic system.
40% studentsanswered this correctly

Important Questions on Solid State
MEDIUM
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
Given is the arrangement of atoms in a crystallographic plane. Which plane correctly represent(s) the drawn structure?
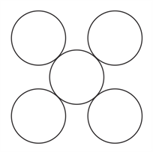
HARD
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
Which mineral can have the silicates skeleton as shown below?
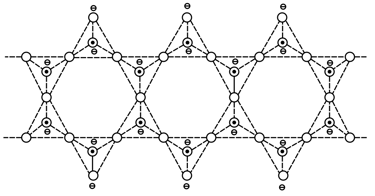
HARD
Chemistry
IMPORTANT
Copper has a face-centred cubic structure with a unit-cell edge length of . What is the size of the largest atom (in pm), which could fit into the interstices of the copper lattice without distorting it?
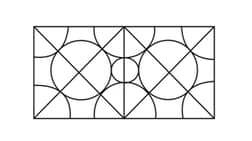
[Hint: Calculate the radius of the smallest circle in the figure.]
HARD
Chemistry
IMPORTANT
A metal cystallises into two cubic phases, f.c.c and b.c.c. whose unit-cell lengths are and . Calculate the ratio of densities of f.c.c. and b.c.c.
Give answer after multiplying with 100 and rounding off to the nearest integer value.
