If the wavelength of the first line of the Balmer series of hydrogen is , the wavelength of the second line of the series should be?

Important Questions on Atomic Physics
The energy level diagram for a hydrogen-like atom is shown in the figure. The radius of its first Bohr orbit is

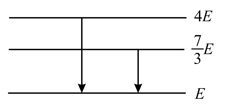
The following diagram indicates the energy levels of a certain atom when the system moves from level to . A photon of wavelength is emitted. The wavelength of photon produced during its transition from level to is . The ratio will be
Energy levels , , of a certain atom corresponding to increasing values of energy, i.e., . If , and are the wavelengths of radiations corresponding to the transitions to , to and to respectively, which of the following statements is correct
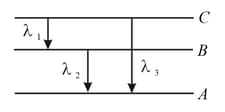
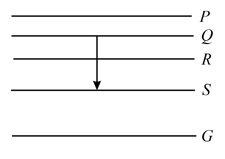
Figure shows the energy levels , , , and of an atom, where is the ground state. A red line in the emission spectrum of the atom can be obtained by an energy level change from to . A blue line can be obtained by the following energy level change
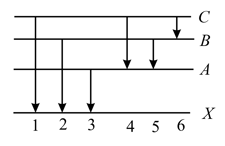
The figure indicates the energy level diagram of an atom and the origin of six spectral lines in emission (e.g. line no. arises from the transition from level to ). The following spectral lines will also occur in the absorption spectrum