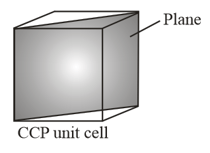
In a hypothetical solid, atoms form lattice with atoms are occupying all the tetrahedral voids () and atoms are occupying all the octahedral voids (). and atoms are of the appropriate size such that there is no distortion in the lattice. Now, if a plane is cut (as shown), then type of voids and their numbers which are present at the cross section would be

Important Questions on Solid State
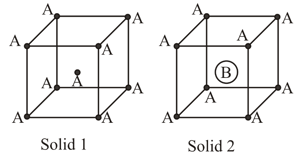
Consider the bcc unit cells of the solids and with the position of atoms as shown below. The radius of atom is twice that of atom The unit cell edge length is more in solid than in . What is the approximate packing efficiency in solid ?
An element crystallises in a face–centred cubic (fcc) unit cell with cell edge a. The distance between the centres of two nearest octahedral voids in the crystal lattice is:
The packing efficiency of the face centered cubic (FCC), body centered cubic (BCC) and simple/primitive cubic (PC) lattices follows the order


