Voids
Voids: Overview
This topic covers concepts, such as Voids and their Types, Octahedral and Tetrahedral Voids, Number and Locations of Tetrahedral and Octahedral Voids in FCC, Triangular Planar Voids, and Cubical Voids.
Important Questions on Voids
Elements and form a compound. The atoms of element arrange in a cubic close packed lattice. The atoms of element occupy half of the tetrahedral voids and all the octahedral voids. The formula of the compound thus formed is [treat and atoms as hard spheres]
A compound is formed by two elements and . The element forms ccp and atom occupies of the tetrahedral voids. The formula of the compound is
A molecule have radius ratio of is more than 0.732. Which of the following void is presented in the molecule.
A molecule have radius ratio of is 0.200 . Which of the following void is presented in the molecule.
Right option for the number of tetrahedral and octahedral voids in hexagonal primitive unit cell are:
The total number of tetrahedral voids in the face centred unit cell is
In a carbon allotrope having lattice, two atoms are at and coordinates. What is the ratio of bond length distance to the edge length of the unit cell?
In a compound, oxide ions are arranged in cubic close packing arrangement. Cations occupy of the tetrahedral voids and cations occupy of the octahedral voids. The formula of the compound is
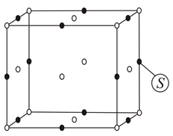
For the structure given below the site marked as S is a :-
The number of tetrahedral voids per atom in a crystal lattice is
An ionic compound is expected to have a tetrahedral structure if lies in the range of
The total number of tetrahedral voids in the face-centred unit cell is _____.
The number of tetrahedral sites per sphere in ccp structure is,
The number of octahedral sites per sphere in fcc structure is
The ratio of close packed atoms to octahedral holes in hexagonal close packing is
The ratio of close packed atoms to tetrahedral holes in cubic packing is
In a solid , having the rock salt structure, atoms form the arrangement and atoms occupy all the octahedral voids. If the atom at body centred octahedral void is missing, then the formula of the compound will be:
In a face centred cubic arrangement of metallic atoms, what is the relative ratio of the sizes of tetrahedral and octahedral voids?
