In an adiabatic expansion of 0.5 mole of an ideal diatomic gas, temperature changes from 32ºC to 20ºC. The change in internal energy of the gas is

Important Questions on Thermodynamics
The following are the P-V diagrams for cyclic processes for a gas. In which of these processes heat is released by the gas ?
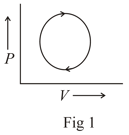
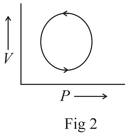
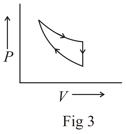
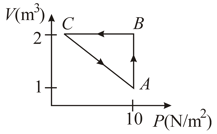
An ideal gas is taken through the cycle as shown in the figure. If the net heat supplied to the gas in the cycle is , the work done by the gas in the process is
One mole of a monatomic ideal gas is taken through a cycle as shown in the diagram. Column-II gives the characteristics involved in the cycle. Match them with each of the processes given in Column-I.
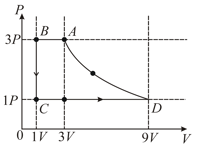
| Column-I | Column-II |
| (A) Process | (p) Internal energy decreases |
| (B) Process | (q) Internal energy increases |
| (C) Process | (r) Heat is lost |
| (D) Process | (s) Heat is gained |
| (t) Work is done on the gas |
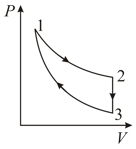
Three processes form a thermodynamic cycle as shown on diagram for an ideal gas. Process takes place at constant temperature (), Process takes place at constant volume, During this process of heat leaves the system. Process is adiabatic and temperature . Work done by the gas during the process is