EASY
9th CBSE
IMPORTANT
Earn 100
In an experiment to separate the components of a mixture of sand, common salt and ammonium chloride, the component which will be removed by filtration is:
(a)sand
(b)common salt
(c)ammonium chloride
(d)none of these
50% studentsanswered this correctly

Important Questions on Is Matter Around Us Pure?
MEDIUM
9th CBSE
IMPORTANT
The correct procedure for heating a mixture of iron filings and sulphur powder is:
EASY
9th CBSE
IMPORTANT
Sulphur powder dissolves carbon disulphide to form yellow coloured solution but solid sulphur reappears by:
EASY
9th CBSE
IMPORTANT
While separating the components of a mixture of sand, camphor and common salt, Mohan added water to the mixture in a beaker and stirred it well. He observed that component that has dissolved in water is:
EASY
9th CBSE
IMPORTANT
Sample 'A' contains iron filings and sulphur powder, Sample 'B' is iron sulphide. Sample 'A' and 'B' are taken on a watch glass and a bar magnet is rolled over both.
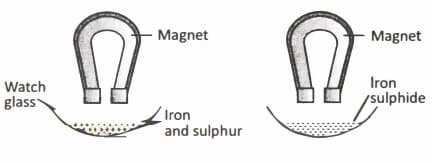
You will observe that:
EASY
9th CBSE
IMPORTANT
In a china dish, of iron filings and of sulphur powder are mixed properly. Suggest a method to separate the individual constituents from its mixture.
EASY
9th CBSE
IMPORTANT
A mixture of sand, ammonium chloride and sodium chloride is dissolved in water and filtered. The filtrate consists of:
EASY
9th CBSE
IMPORTANT
To separate a mixture of iron filings, sulphur powder and iron sulphide, which sequence of methods should be used?
EASY
9th CBSE
IMPORTANT
A mixture contains iodine, ammonium chloride and sand. Only iodine and ammonium chloride sublime. Only iodine dissolves in carbon tetrachloride.
How will you separate the three components? Sequence of steps will be:
