HARD
JEE Main
IMPORTANT
Earn 100
In diamond, carbon atoms occupy lattice points, as well as alternate tetrahedral voids. If the edge length of the unit cell is , then the diameter of the carbon atom is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Solid State
MEDIUM
JEE Main
IMPORTANT
EASY
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
Copper has a face-centred cubic structure with a unit-cell edge length of . What is the size of the largest atom (in pm), which could fit into the interstices of the copper lattice without distorting it?
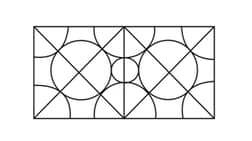
[Hint: Calculate the radius of the smallest circle in the figure.]
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
The density of a pure substance '' whose atoms are packed in a cubic-close packed arrangement is . If atoms can occupy tetrahedral void and all the tetrahedral voids are occupied by '' atoms, what is the density of resulting solid in Atomic mass and atomic mass .
